2022-04-08 12:10:46
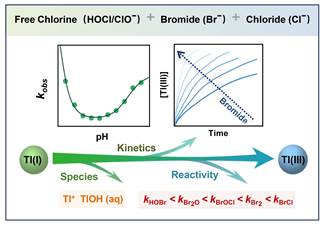
Professor HUANGFU Xiaoliu’ team from the College of Environment and Ecology, has newly published a study on kinetics of thallium(I) oxidation by free chlorine inEnvironmental Science & Technologyas asupplementary cover. In this study, the role of Br¯ on the kinetics of Tl(I) oxidation in chlorinated waters was investigated and the species-specific rate constants of Tl(I) with reactive bromine species werequantified. The model predictions for Tl(I) oxidation under conditions typical for drinking water and wastewaters treated by chlorine were developed. This study refine our knowledge regarding the species-specific reactivity of TI(I) with bromine species and will be useful for further prediction of thallium mobility in chlorinated waters containing bromide.
https://doi.org/10.1021/acs.est.1c06901


Graphic Abstract
Abstract
The oxidation of thallium (Tl(Ⅰ)) to Tl(ⅠII) by chlorine (HOCl) is an important process changing its removal performance in water treatment.However, the role of bromide (Br¯), a common constituent in natural water, on the oxidation behavior of Tl(I) during chlorination, remains unknown. Our results demonstrated that Br¯ was cycled and acted as a catalyst to enhance the kinetics of Tl(I) oxidation by HOCl over the pH range of 5.0−9.5. Different Tl(I) species (i.e., Tl+and TlOH) and reactive bromine species (i.e., HOBr/BrO¯, BrCl, Br2O, and BrOCl), were kinetically relevant to the enhanced oxidation of Tl(Ⅰ).The oxidation by free bromine species became the dominant pathway even at low Br¯ level of 50 μg/L for a chlorine dose of 2 mg Cl2/L.It was found that the reactions of Tl+/BrCl, Tl+/BrOCl, and TlOH(aq)/HOBr dominated the kinetics of Tl(I) oxidation at pH< 6.5, pH 6.5-8.0, and pH > 8.0, respectively. The species-specific rate constants forTl+reacting with individual bromine species were determined and decreased in the order: BrCl > Br2> BrOCl> Br2O > HOBr. Overall, the presented results refine our knowledge regarding the species-specific reactivity of TI(I) with bromine species and will be useful for further prediction of thallium mobility in chlorinated waters containing bromide.
Aims
This work aimed to (i) investigate the bromination kinetics of Tl(I) over a wide pH range; (ii) examine the role of Br¯ on the kinetics of Tl(I) oxidation in chlorinated waters; (iii) quantify the species-specific rate constants of Tl(I) with reactive bromine species; (iv) develop the model predictions for Tl(I) oxidation under conditions typical for drinking water and wastewaters treated by chlorine.
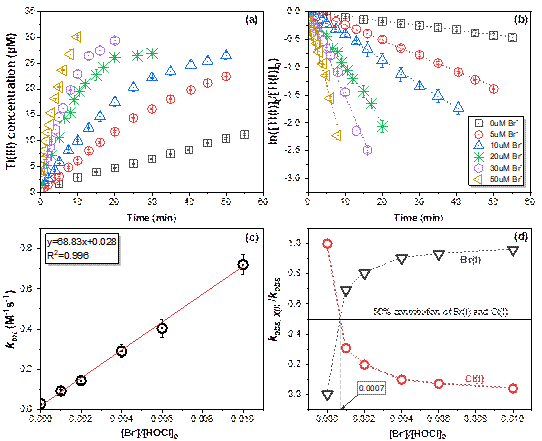
Figure. Influence of Br−on the oxidation of Tl(I) by HOCl. (a) Concentration change of Tl(Ⅲ) as afunctionof time. (b)Pseudo-first-order kinetics fitting for the Tl(I) oxidation by HOCl. (c)ktotas a function of[Br−]/[HOCl]0ratio. (d) Relative contributions of bromide and chlorine to the![]() as a function of [Br−]/[HOCl]0ratio.
as a function of [Br−]/[HOCl]0ratio.
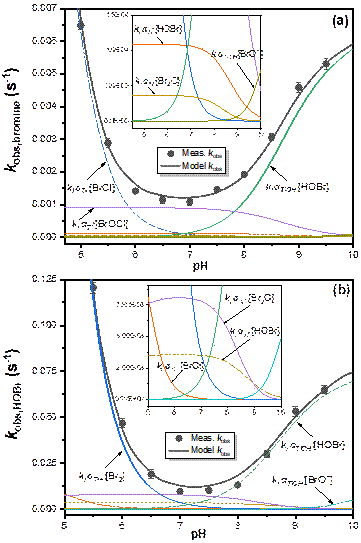
Figure. (a)Pseudo-first-order rate constants of Tl(I) oxidation by bromine (![]() ) as a function of pH during chlorination.(b) Pseudo-first-order rate constants of Tl(I) oxidation by HOBrT(
) as a function of pH during chlorination.(b) Pseudo-first-order rate constants of Tl(I) oxidation by HOBrT(![]() ) as a function of pH. Error bars denote 95% confidence intervals (smaller than symbols if not shown). The black solid lines denotes model fits.
) as a function of pH. Error bars denote 95% confidence intervals (smaller than symbols if not shown). The black solid lines denotes model fits.
Environmental applications
The redox kinetics of Tl determines its principal redox state, which in turn, has implications for the removal performance of this highly toxic trace element. While chlorine was demonstrated to be an efficient oxidant for Tl(I) in water treatment,this study was the first to highlight the importance of Br−on influencing the kinetics and pathway of Tl(I) transformation during chlorination. As shown here, the oxidation of Tl(I) by chlorine was significantly enhanced through a Br−-catalyzed process. Only a few μg/L Br−were necessary to change the pathway of Tl(I) oxidation from chlorine to the subsequently formed bromine species as a result of Br−oxidation by chlorine. The species-specific rate constants of the key reactions of Tl(I) with reactive bromine species were identified, the extent to which those free bromine species influenced Tl(I) oxidation was of practical importance for guiding the trace thallium treatment in chlorinated drinking water (DW) or wastewater(WW).